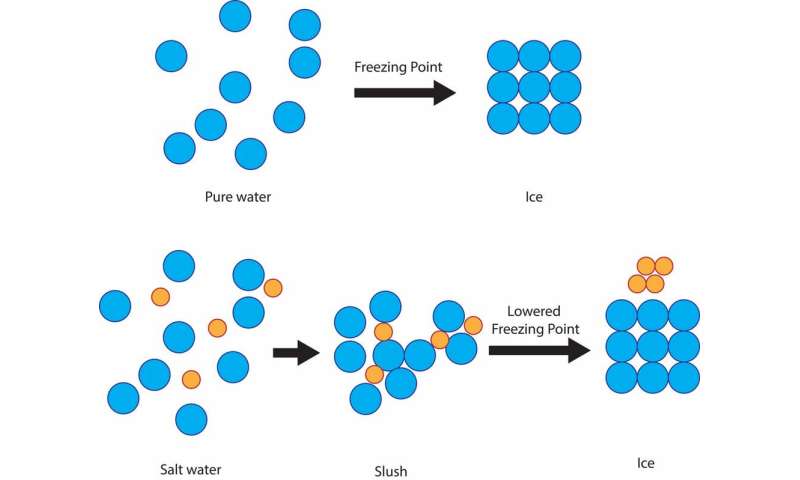
Salt sugar and antifreeze all lower the freezing point of water. A chemical change between the water and other substance keeps it from freezing at 0 degrees celsius 32 degrees fahrenheit.
Chemistry 104 Molecular Weight By Freezing Point Depression
To comprehend freezing point depression you must first understand freezing point.

Lowering the freezing point of water. Scientists have found though that you can raise the freezing point of supercooled pure water by using electricity adding alcohol or adding soot or testosterone. Remove the test tube from the bath stir vigorously and note the constant temperature during the time that ice and water ate both present. Addding almost any impurity to water will lower the freezing point.
A chemical change between the water and other substance keeps it from freezing at 0 degrees celsius 32 degrees fahrenheit. You may try different concentrations of the same salt to see the effect on waters freezing point or you could try the same concentration of different substances. Figure 1 freezing point apparatus in ice salt water bath.
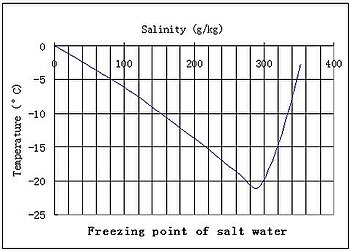
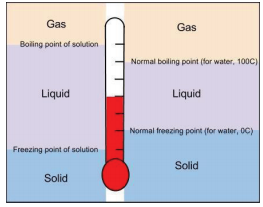
An important property that you can calculate from molality is freezing point depression. Adding a salt especially nacl is a popular way. If this new freezing point is lower than the outside temperature.
You can lower the freezing point of water by adding a solute such as salt but raising the freezing point isnt quite as easy. Experiment 12 freezing point of solutions. It is meant to keep the engine from freezing and from overheating because adding something to water lowers the freezing point and raises the boiling point.
Today principalities multiply sodium on icy roads in order to melt the snow. Thats why you sprinkle salt on icy sidewalks. The salt mixes with the ice and lowers its freezing point.
When you add solute to a solvent it lowers its freezing point. Of all ways adding anti freeze which is usually a glycol solution car. In actuality the salt is merely depressing the freezing point of the water allowing the roads to remain ice free whilst the temperatures are below 0 certifications centigrade.
Either way you will have.
 Salt Doesn T Melt Ice Here S How It Actually Makes Winter
Salt Doesn T Melt Ice Here S How It Actually Makes Winter
:max_bytes(150000):strip_icc()/the-freezing-point-of-water-609418_FINAL-01f50f5f4f7d4a39854bebcc59df1aa4.gif) Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcrz26jr Hdvfiyvz0 Hmwbxaggmqehyom2t3dyndkgid Gkjbd Usqp Cau
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcrz26jr Hdvfiyvz0 Hmwbxaggmqehyom2t3dyndkgid Gkjbd Usqp Cau
 Chemistry Of Ice Cream Making Lowering The Freezing Point Of
Chemistry Of Ice Cream Making Lowering The Freezing Point Of
 Chemistry Of Ice Cream Making Lowering The Freezing Point Of
Chemistry Of Ice Cream Making Lowering The Freezing Point Of
 Freezing Point Depression Wikipedia
Freezing Point Depression Wikipedia
 How Do Solutes Affect A Solution S Boiling Point Ppt Download
How Do Solutes Affect A Solution S Boiling Point Ppt Download
What Is The Freezing Point Of Sugar Water How Is This Determined
 What Is Freezing Point Depression How It Works With Videos
What Is Freezing Point Depression How It Works With Videos
Freezing And Refrigerated Storage In Fisheries 2 Influence Of
Science Fair Projects The Effect Of Salt And Sugar On The
 Chemistry Of Ice Cream Making Lowering The Freezing Point Of
Chemistry Of Ice Cream Making Lowering The Freezing Point Of
 Why Does Salt Lower The Melting Point Of Ice Worldofchemicals
Why Does Salt Lower The Melting Point Of Ice Worldofchemicals
 Unit Chemical Interactions Chapter 8 Solutions When Substances
Unit Chemical Interactions Chapter 8 Solutions When Substances
Https Arc Nesa Nsw Edu Au Files Science Act6 Ws3 Pdf
 Photons Powers And Primary Colors Freezing Point Depression
Photons Powers And Primary Colors Freezing Point Depression
 Solved 7 Suppose You Are Making Ice Cream With An Old Fa
Solved 7 Suppose You Are Making Ice Cream With An Old Fa
 Changing Melting And Boiling Points Putting Salt On Sidewalks And
Changing Melting And Boiling Points Putting Salt On Sidewalks And


Tidak ada komentar:
Posting Komentar